A breakthrough innovation in allogeneic cell therapy for tissue repair
Scarcell Therapeutics is developing a highly differentiated cell therapy solution
Regenerative therapies using tissue-engineered products such as cell therapies are becoming
increasingly attractive because they have the potential to treat certain diseases for which
conventional drug therapies are suboptimal. However, most cell therapies developed to date present
toxicity issues that affect their overall efficacy and/or are very expensive to produce, therefore limiting patient access.
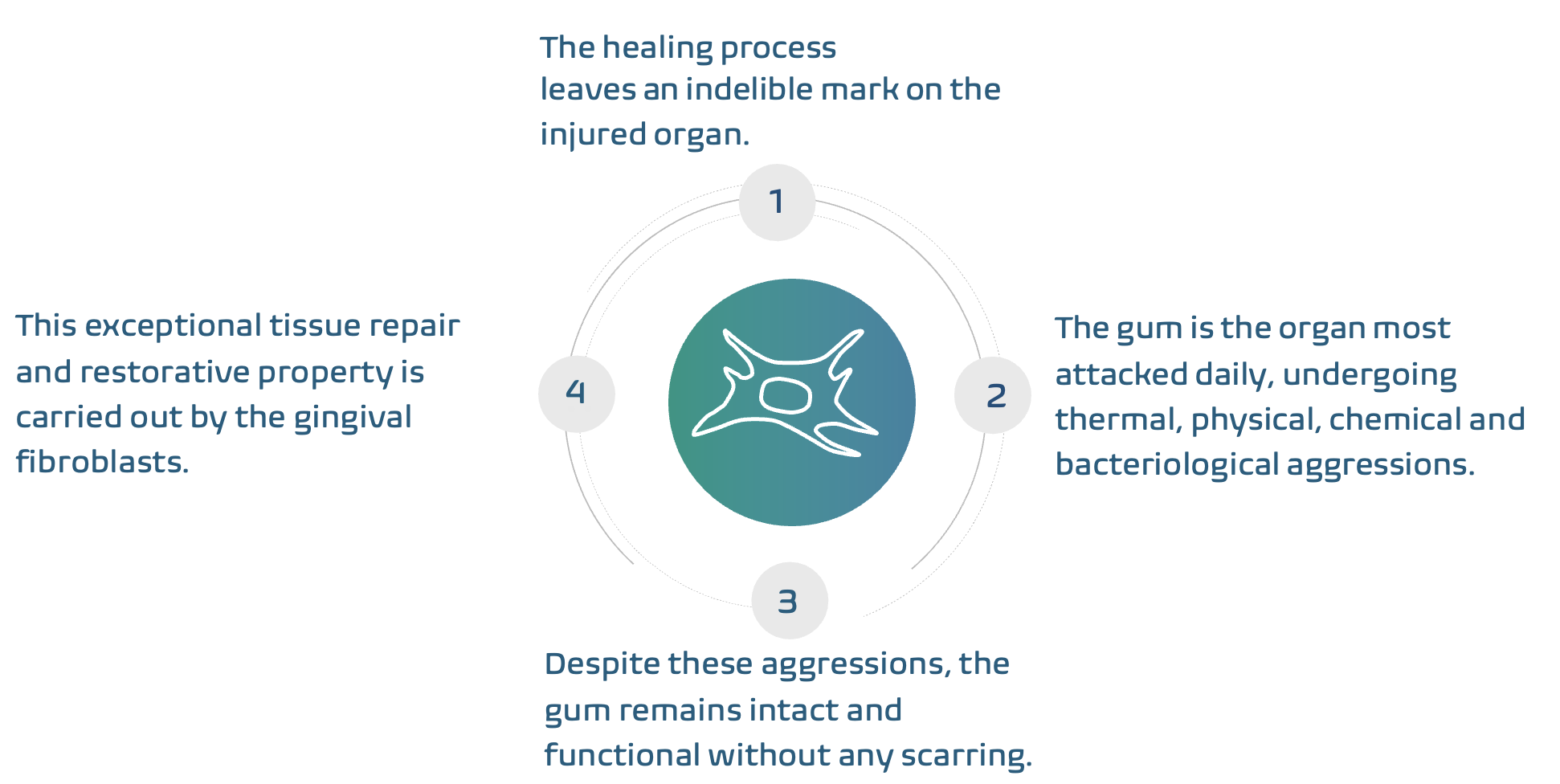
Scarcell Therapeutics was founded on the principle that certain cells in human gum tissue (human
Gingival Fibroblasts (hGFs)) have significant regenerative properties that are naturally “embryo like”, i.e.,
that have the ability to regenerate an extracellular matrix, modulate the host immune response and
promote rapid and scarless healing.
Numerous preclinical studies conducted by Scarcell Therapeutics have demonstrated that hGFs have
a clinically significant capacity for tissue repair whilst maintaining a high degree of immune-tolerance
and safety.
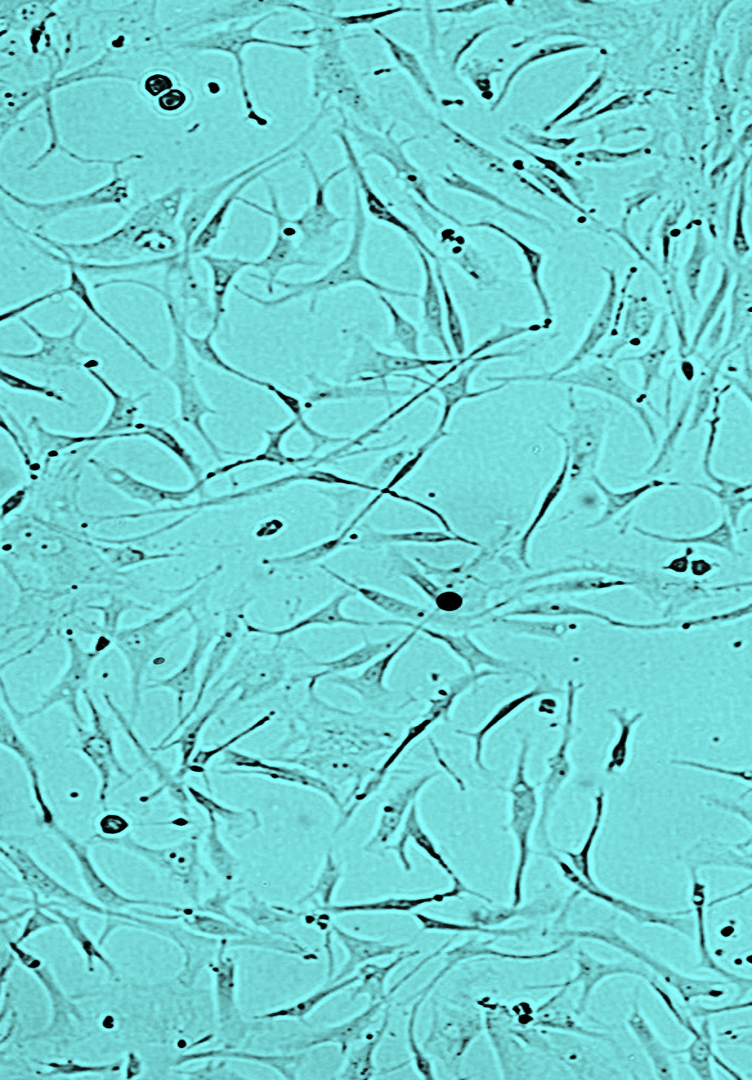
GINGIVAL FIBROBLASTS
a unique combinationOur breakthrough bioproduction process
Highly effective, straightforward, and scalable bioproduction under GMP conditions at considerably lower costs
The other advantage of hGFs is their ability to be produced efficiently and therefore at a fraction of the cost under biologically nontoxic conditions. Scarcell Therapeutics has developed a specific manufacturing process to ensure production of safe and affordable hGF product doses to increase patient access to this innovative therapy.
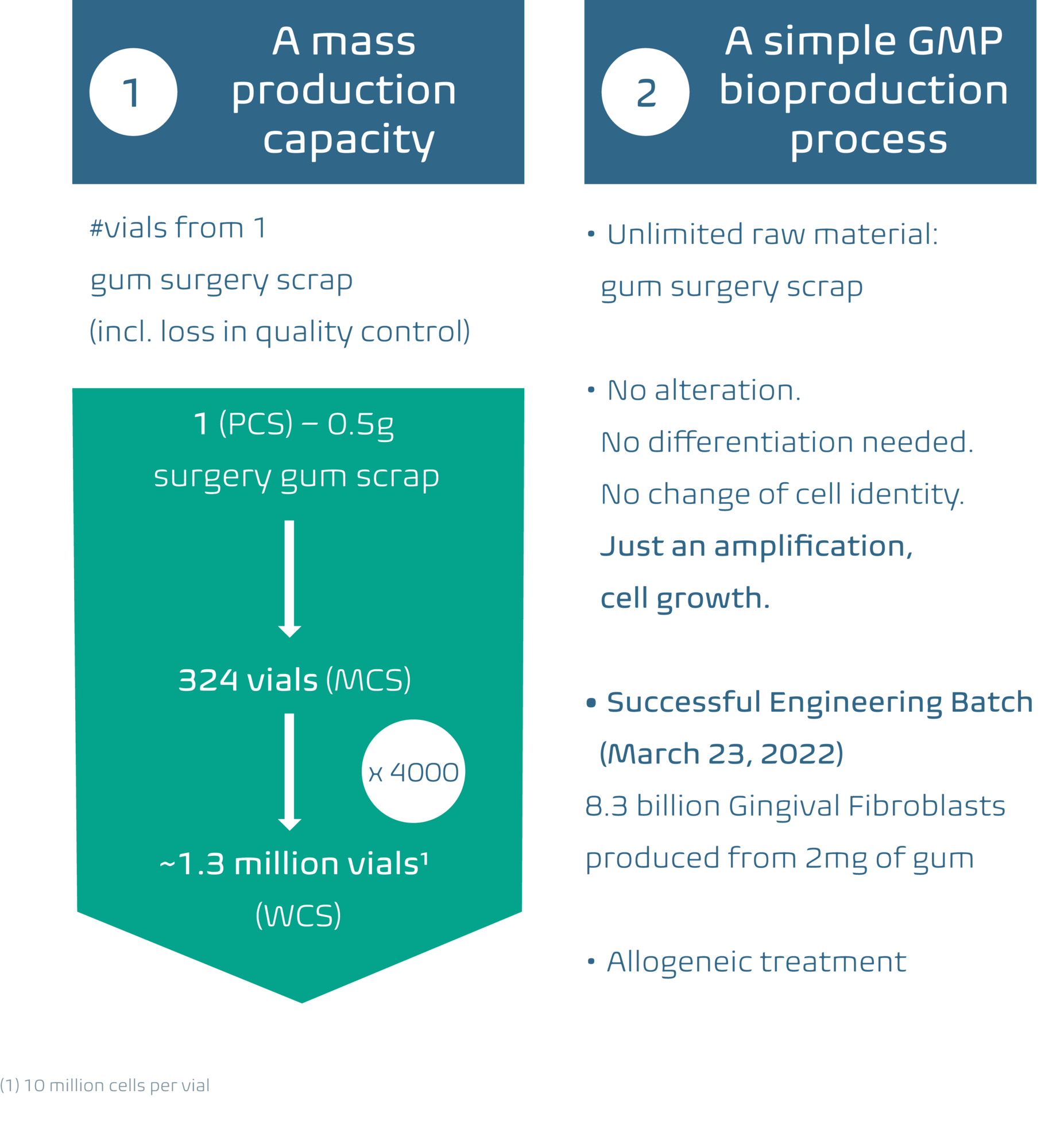
• Scarcell Therapeutics' GMP bioproduction process results in a highly competitive cost of production per therapeutic dose, lowering it by a factor of 10 to 300 compared to current stem cell therapies or in those in development (i.e., MSCs or iPS).
• The hGF cell therapy production process involves no cell alteration:
• No cell differentiation (cells are already differentiated by nature).
• No change of cell identity.
• Only cell selection and expansion.
• Scarcell Therapeutics generates 1.3 million therapeutic doses of hGF from a single unit of surgery gum scrap.
• As a result, Scarcell Therapeutics intends to be able to propose an affordable selling price per treatment.
Our publications
Intellectual Property
Scarcell Therapeutics has secured a pioneering IP portfolio of patents covering its proprietary gingival fibroblasts as a cell therapy product and the first clinical indication to be targeted worldwide.
Further innovative indications are under investigation.
Impacts
Technological impact
• Innovative bioproduction using non-modified human GFs decreases production costs to allow greater patient access to effective allogeneic cell therapy
Clinical impact
•A unique combination of anti-inflammatory and tissue repair properties via immune tolerance may allow for new treatments to be created for many diseases, such as inflammatory and tissue repair disorders.
Social impact
• Scarcell Therapeutics' goal is to provide an effective new regenerative therapy at a lower cost
Environmental impact
• Environmentally friendly bioproduction process
• May reduce drug consumption